Presentation of the Bachelor Biotech Engineering
The Bachelor Biotech Engineering Diploma, in accordance with CTI Opinion no. 2020/11-4, trains assistant engineers in biology-biochemistry-biotechnology, conferring the degree of Licence (Bac+3) and certified by the RNCP (no. 37688) for the needs of industrial sectors.
Engineering assistants at the service of industry
The Bachelor Biotech Engineering is a diploma offering three different courses: industrial production, methods & prevention and diagnostics & bioinformatics. The course consists of 4 common academic semesters and 2 semesters (16+24 weeks) of internships. The first year includes a common base of methodological, scientific and technical knowledge and a specialization according to the courses in the second and third years.


Pathway 1: Industrial production
The aim of this program is to train production engineering assistants. They are involved in perfecting industrial processes, and monitor production in conjunction with the quality department and team leaders: conducting manufacturing operations in the pharmaceutical, cosmetics and food sectors. Find out more about the Bachelor Biotech Engineering – Industrial production course

Pathway 2: Methods and prevention
The aim of this pathway is to train assistant methods engineers who act as a link between the design office and the workshop. They are responsible for optimizing the various jobs, taking all parameters into account: regulatory standards, quality, costs and deadlines. Find out more about the Bachelor Biotech Engineering – Methods and prevention course

Pathway 3: Diagnostics and bioinformatics
The aim of this program is to train assistant engineers in diagnostics and bioinformatics. They are responsible for implementing and developing diagnostic techniques and applying bioinformatics tools. They work alongside researchers and engineers in research and development (RD) or design offices. Find out more about the Bachelor Biotech Engineering – Diagnostics and bioinformatics course
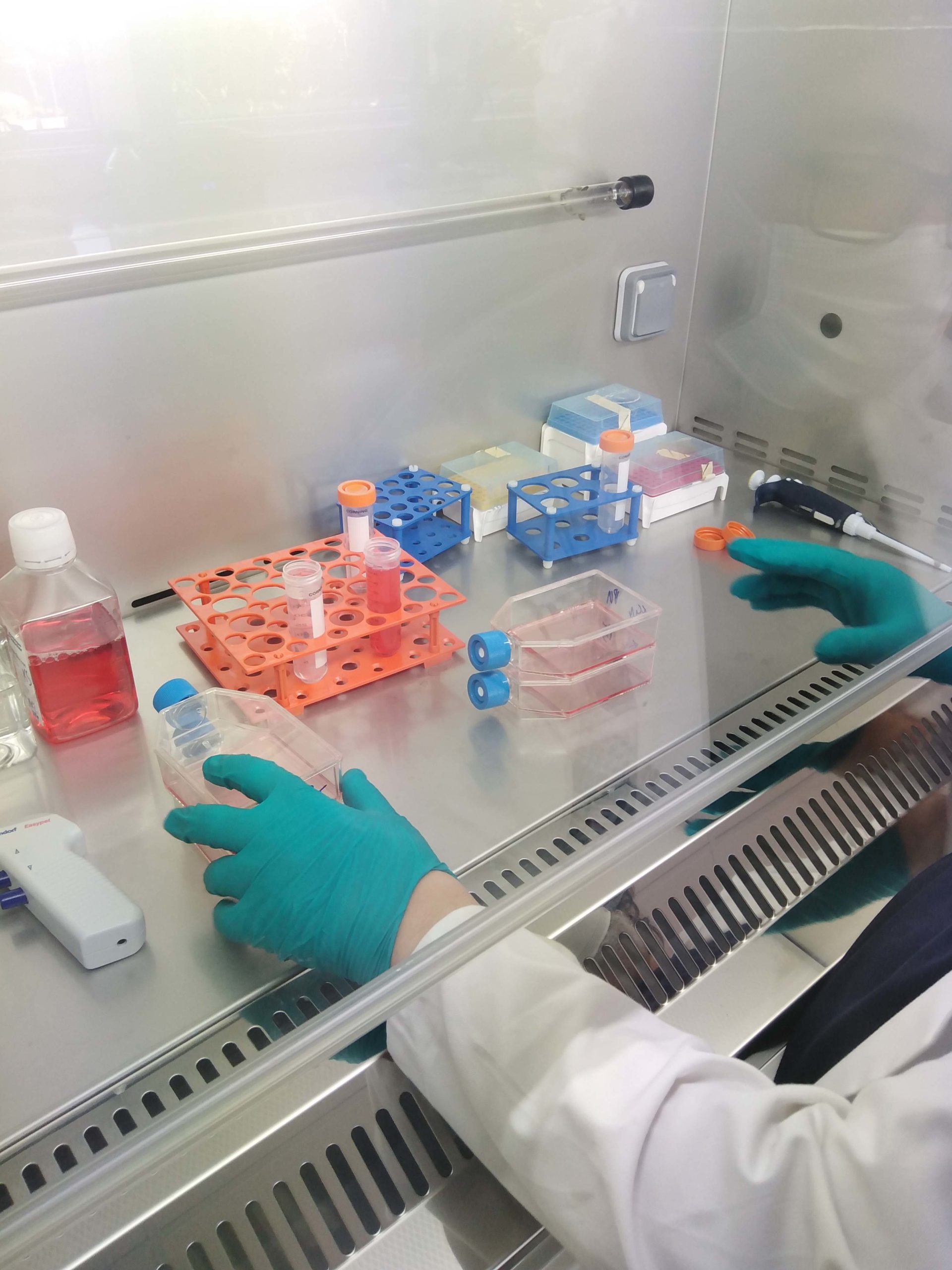
1000 m² where engineering assistants, engineers, experts and researchers rub shoulders
The teaching methodology will be based on the technical platform of the EBI; its laboratories include state-of-the-art equipment: molecular biology, cell culture, microbiology, formulation, production, analytical and process pilots. This practical approach will enable you to handle and master all types of equipment, integrating different approaches to optimization, quality and risk management.

Skills developed
Scientific and technical knowledge in bioprocesses, diagnosis, prevention, quality management with an organizational, regulatory and data analysis dimension. This multidisciplinary approach will be the basis of this training in coherence with the three proposed courses.

Active teaching methods applied to real-life industrial issues
The training is oriented towards a perfect mastery of the scientific and technical bases, of the critical parameters of the production/diagnostic systems by integrating at the same time the approaches of quality, risk management, qualitative and quantitative characterization methods. This approach is entirely supported by an active pedagogy where the student is the actor of his learning: project and problem-based learning (PBL).








